Echinacea is a perennial herb of the composite family echinacea, which is included in the “United States Pharmacopoeia” as one of the medicinal base sources of echinacea. It is naturally distributed in the east-central United States and southern Canada, and historically is a traditional medicine of North American Indians. Pharmacological studies have shown that echinacea has immune-enhancing, anti-inflammatory and antiviral effects. It has become one of the most commonly used immune-enhancing herbs in the world and is used as a dietary supplement. Echinacea’s main effective components, including caffeic acid derivatives, alkyl amide, polysaccharide and the volatile oil and so on, including Echinoside, a type of caffeic acid derivative, were representative material of Echinacea herb, can inhibit tumor cells, anti-aging, diabetes and other benefits, but because of its content is low and difficult to separate, higher requirements for the instrument, measurement is relatively difficult, At present, the separation and analysis are mainly carried out by reverse high-performance liquid chromatography(HPLC) gradient elution.
- Test materials
The echinacea plants used in this experiment were harvested in August 2020 at the peak flowering period of echinacea and stored in four parts: stem, leaf, root and head, after being dried in shade at -20 ℃. The equipment is Agilent1100 high effect liquid phase chromatograph, AgilENT-G-1315B-DAD purple external detector, Agilent322A true space in-line degassing machine, HPChem-Station color spectrometer, DFY-400 swing high-speed universal crusher, T2000Y electron analysis, SK82001HC ultrasonic wave cleaning instrument, TG-16W centrifugal machine.
Reagents include acetonitrile, methanol, phosphoric acid, glacial acetic acid, ethanol and other chromatographic pure reagents, water is pure water; Echinoside Powder(From Rainbow Biotechnology Co., LTD., content ≥ 98% by HPLC).
2. Test Method
Preparation of mobile phase
Placement of 0.1% phosphoric acid solution: accurately remove 1.00ml phosphoric acid solution into a volumetric flask, and use pure water to keep the volume to 1000mL. Shake it well, and ultrasonic for 5min to make the solution fully mixed. Then filter it with a vacuum filter device, and then ultrasonic to remove bubbles. The configuration of 0.1% glacial acetic acid solution: accurately remove 1.00ml glacial acetic acid solution into a volumetric bottle, use pure water to keep the volume constant to 1000mL, shake well, ultrasonic for 5min to make the solution fully mixed, then filter with a vacuum filter device, and ultrasonic to remove bubbles.
Preparation of standards
The standard echinacoside 0.01g was precisely weighed, dissolved in 70% ethyl alcohol, and then in a constant volume of 10 mL bottle, prepared into 1mg·mL-1 reference substance mother liquor. Accurately absorb 1mL of the mother solution of Echinoside reference substance, put it into a labeled 10mL volume bottle, dilute it with 70% ethyl alcohol to the degree of accuracy, shake well, prepare 0.1 mg·mL-1 reference substance storage solution, stand, then filter with microporous membrane (0.45 μm), the filtrate is the standard solution of Echinoside.
Solution preparation
The different echinacea materials were dried and pulverized separately and sifted through 100 mesh. Weigh a certain amount of each sample medicinal powder, weigh it accurately, add 70% ethyl alcohol, constant volume to 10 mL, label, shake well, ultrasonic (40kHz) for 20min, and cold soak in a refrigerator at 4 ℃ for 24h. The extraction was followed by ultrasonic (40kHz) for 20 min and centering (3500r·min-1) for 10min. Absorb the supernatant and add 70% ethanol to 25 mL, shake well and place, filter with microporous membrane (0.45μm), filtrate is the test liquid of each sample.
Data analysis
SPSS17.0 software was used for significance analysis. P≤ 5% indicated significant difference. Excel2007 software for standard curve making and relative standard deviation (RSD) calculation; For each group of data, three groups of parallel tests were carried out to get the average value.
3. Results and analysis
Optimization of chromatographic column
The mobile phase was acetonitrile-0.1% phosphoric acid (21∶79, v/v). The detection wavelength was 330nm, the column temperature was 30 ℃, the injection volume was 10μL, and the flow rate was 1.0 mL·min-1. Compared with Agilent (tc-c185 μm 4.6 mm×250mm) and Diamonsil (5μm C18, 4.6 mm×250mm), the separation of echolin standard solution was better by standard solution analysis. The results showed that Agilent (TC-c185 μ m4.6 mm×250 mm) had a better separation effect on pineoside.
Optimization of mobile phase and elution mode
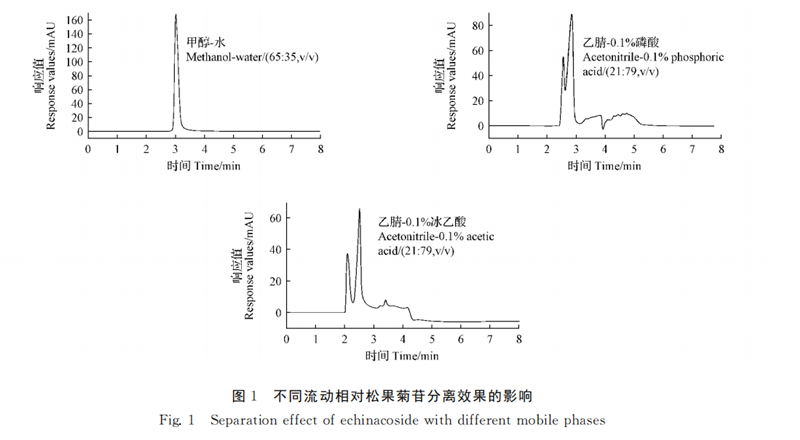
Agilent (TC-C185μm 4.6 ×250mm) was selected, the detection wavelength was 330nm, the column temperature was 30 ℃, the injection volume was 10μL, and the flow rate was 1.0 mL·min-1. The separation of the standard solution of echinacoside with the mobile phase of methanol-water (65∶35, V/V), acetonitrile-0.1% phosphoric acid (21∶79, V/V) and acetonitrile-0.1% glacial acetic acid (21∶79, V/V) was investigated. The results showed that the separation rate of echinacoside was better when methanol-water (65∶35, V/V) was used as the mobile phase (FIG. 1).
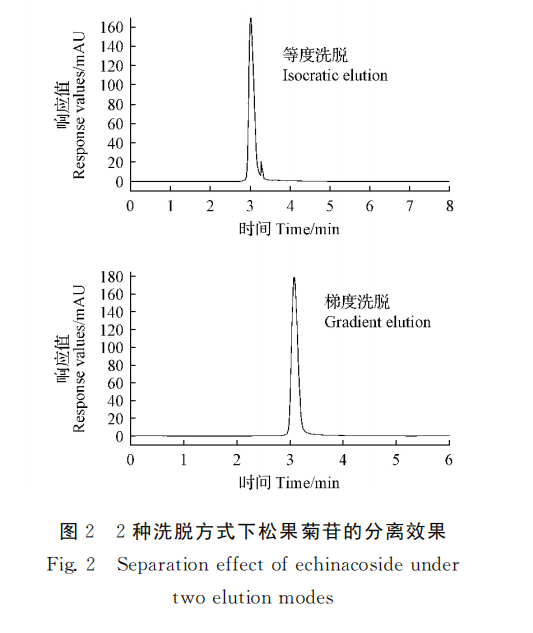
The separation of pinhioside by mobile phase methyl alcohol-water isodegree washing (65∶35, V/V) and gradient washing were compared. The results showed that the standard solution of echinacoside was separated well under gradient elution, and the specific elution procedure was as follows: The elution gradient of mobile phase water (A) and methanol (B) was 58% B (0 ~ 1 min, v/V), 58% ~ 62% B (1 ~ 2min, V/V), 62% ~ 65% B (2 ~ 4min, V/v), 65% ~ 58% B (4 ~ 8min, V/V). The separation degree of pineoside was the best (FIG. 2).
Optimization of detection wavelength
The absorbance of echinacoside at 254, 327, 330 and 360nm was compared. Finally, the absorbance of echinacoside was higher when the wavelength was 330nm.
Optimization of column temperature: The separation effect of echinacoside at column temperature of 20, 25, 30, 35℃ was compared. The results showed that the separation effect of the echinacoside chromatographic peak was good when the column temperature was 30 ℃.
The chromatographic conditions
The optimized chromatographic conditions were as follows: chromatographic column AGI-lent (TC-c185 μ m4.6 mm×250mm), mobile phase methanol-water, flow rate 1.0 mL·min-1, detection wavelength 330nm, column temperature 30 ℃, injection volume 10μL, gradient elution.
Precision Test
The standard solution of echinacoside was injected 9 consecutive times to calculate the RSD of the peak area. The results showed that the peak area RSD of echinacoside was 0.81%, indicating that the precision of the instrument was good.
Stability Test
Prepare 0.1 g of the same echinacea sample powder and the sample solution, and inject 10μL at 0, 2, 4, 6, 8, 10, 12 and 24h, respectively. Analyze the sample for 8 consecutive times according to the chromatographic conditions mentioned above, and calculate the RSD value of peak area. RSD of pinocyside was 0.50%, and the relative standard deviation was within 5%. The results showed that the tested solution was stable within 24h.
Repeatability Test
Prepare 6 samples of the same echinacea sample powder, 0.1 g for each sample and the solution of the sample for sample injection and analysis under the chromatographic conditions mentioned above. The RSD of the echinacoside peak area was 2.23%. The results showed that the method had good repeatability.
Recovery Test
The last 6 echinacoside powders with known concentrations were accurately weighed and divided into high and low concentrations. Relevant data showed that when measuring the recovery rate, the appropriate dosage phase should be 0.5 ~ 2.5 times of the content of the test group. Adding 10μg·mL-1 pinoside, the content of the sample was 2 times of the sample content. According to the designed solution and chromatographic conditions of the external standard method to calculate the concentration, calculate the recovery of each compound and the recovery of RSD value.
Marked recovery rate (%) = (measured value of marked test sample – the measured value of sample)/scalar ×100.
The recovery rate of echinacoside was calculated by the above formula. When measuring the recovery rate, the allowable limit of recovery rate is 80% ~ 120%, and the relative standard deviation is within 5% when the content of the tested component is less than or equal to 1mg·L-1. The results were shown in Table 3, which showed that the recovery of echinacoside ranged from 88.03% to 97.88%, and the average recovery was above 93%. RSD was 2.17% at low concentration, 0.38% at high concentration and 1.28% at normal level, which met the requirements of paper submission. It showed that the recovery rate was good and the method was feasible.
Determination of sample content
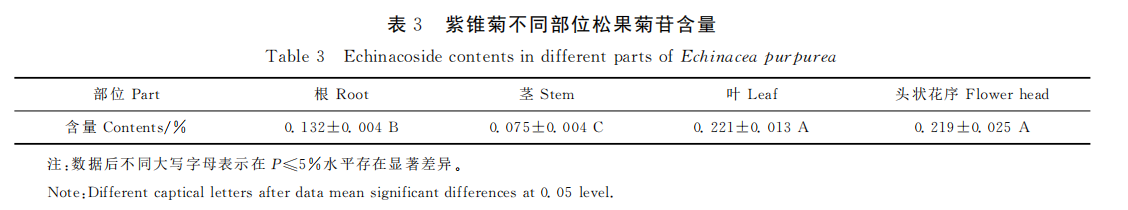
The root, stem, leaf, inflorescence and inflorescence peels of echinacea were precisely measured with 0.1g powder, and the test solution was prepared according to the method described above and the samples were analyzed under the specified chromatographic conditions, and the content of echinacoside in each part was calculated by the external standard method. Each sample was measured three times in parallel. Echinacea showed different contents of echinacoside in different parts. The content of echinacoside in leaves was the highest (0.221%), the content of echinacea in the head was 0.219%, which was not significant but significantly higher than that in roots (0.132%), and the content of echinacoside in stems was the lowest (0.075%).
The separation and analysis of echinacoside are difficult and there is no unified method. Test by comparing the different chromatographic column, column temperature, detection wavelength, mobile phase and the way of echinacea elution glycosides quantitative analysis, the influence of optimized echinacea quantitative analysis method, the optimized separation analysis method to improve the chromatographic peak shape, after methodology investigation show that the quantitative analysis method is stable, accurate and reproducible, The method is simple and feasible. The optimized HPLC was performed on an Agilent (tc-c185 μ m4.6 mm×250mm) column with a detection wavelength of 330nm, column temperature of 30 ℃, a sample volume of 10μL, mobile phase was methanol-water, flow rate of 1.0 mL·min-1, gradient elution.
The results showed that echinacoside content in echinacea family various and Echinacea angustifolia, and Echinacea pallida have a high content than that of Echinacea purpurea, and echinacoside content in the above-ground part was higher than that in the underground part. BINNS reported that echinacea roots produced in Canada varied in 0% ~ 0.054%, and echinacea roots contained a high content of echinacoside, up to about 0.6%. The results showed that the content of echinacoside in the roots of three-year-old white Echinacea was 0.596%. According to this research, the content of echinacoside of echinacea purpurea varied greatly in different parts ranging from 0.075% to 0.221%, leaves and heads was higher than that in roots, and the basal stems were the lowest. Echinacea extract manufacturers in China and India currently have echinoside concentrations ranging from 0.01% to 0.23%, depending on the origin.
- Dandelion Extract: What It Is, Benefits, Uses and Side Effect - April 23, 2024
- Is Berberine Extract Help For Weight Loss? - April 11, 2024
- Why Is Pysllium Husk Powder A Popular Meal Replacement Ingredient? - April 3, 2024